Saxenda® (liraglutide 3 mg) is a once-daily glucagon-like peptide-1 (GLP-1) analogue with 97% similarity to naturally occurring human GLP-1, a gut hormone involved in appetite regulation.2
Saxenda® (liraglutide 3 mg), a once-daily glucagon-like peptide-1 (GLP-1) analogue involved in appetite regulation, lowers body weight through decreased food intake. Saxenda® does not increase 24-hour energy expenditure.
Furthermore, Saxenda® stimulates the release of insulin and suppresses glucagon secretion in a glucose-dependent manner, when needed. These effects can lead to a reduction in blood glucose.3
Saxenda® binds to and activates GLP-1 receptors in the brain that are involved in the regulation of appetite. They are processed by the brain and translated into feelings of satiety or hunger and thereby control food intake.4
Acute pancreatitis is considered an identified risk for all GLP-1 receptor agonists.5
In phase 2 and 3a studies from the Saxenda® clinical development programme, there was a numerical imbalance in the number of cases of acute pancreatitis in people with obesity treated with Saxenda® compared to placebo. In the SCALETM Obesity and Prediabetes trial there were a total of 13 confirmed cases of acute pancreatitis (12 cases in the Saxenda® group [0.4% of patients; 0.26 events per 100 PYR] and 1 case in the placebo group [<0.1% of patients; <0.1 events per 100 PYR]).6
This imbalance is consistent with that observed with Victoza® (liraglutide up to 1.8 mg) in type 2 diabetes.
Based on the totality of information available there is no indication of a causal relationship between treatment with Saxenda® and pancreatic cancer.
Additional information:
In a joint statement published in the New England Journal of Medicine, the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) concluded that assertions concerning a causal association between incretin-based therapies (including Victoza®) and pancreatitis are inconsistent with the current data available, based on a comprehensive review of available data regarding the pancreatic safety of incretin-based drugs. The FDA and EMA have not reached a final conclusion at this time regarding a causal relationship.7
Across the SCALETM clinical development programme, Saxenda® was generally well tolerated.6 In line with previous liraglutide trials, the most common side effects were related to the gastrointestinal system, such as nausea, vomiting, diarrhoea and constipation, and were transient.8, 9
These reactions were usually mild or moderate, and transitory with the majority disappearing during continued treatment, usually after four weeks.
Nausea is a common side effect with all GLP-1 receptor agonists and is typically seen at the beginning of treatment. The underlying mechanisms of this association are not clearly understood.
Novo Nordisk is committed to patient safety and continuously monitors for adverse events in relation to the use of its products.
Based on an assessment of the totality of safety data available, there is no indication of a causal relationship between thyroid cancer and treatment with Saxenda®.
MTC
The US label for Saxenda® includes a boxed warning for thyroid C-cell tumours, which includes MTC. The EU label also includes a warning for thyroid disease.10 Based on the 120-day safety update submitted to the FDA by Novo Nordisk in April 2014, no cases of MTC were reported with Saxenda® treatment in the phase 2 and phase 3a SCALETM clinical development programme.7 As noted in the US label, cases of MTC in patients treated with liraglutide have been reported in the post-marketing period; the data in these reports are insufficient to establish or exclude a causal relationship between MTC and liraglutide use in humans. 3
Papillary thyroid carcinoma
Papillary thyroid cancer is included under section 6.1 Adverse Events, Clinical Trials Experience, within the US label. In Saxenda® clinical trials, papillary thyroid carcinoma confirmed by adjudication was reported in seven (0.2%) of 3291 people treated with Saxenda® compared with no cases among 1843 people treated with placebo. Four of these papillary thyroid carcinomas were less than 1 cm in the greatest diameter, and four were diagnosed in surgical pathology specimens after thyroidectomy prompted by findings identified prior to treatment.7
Novo Nordisk is committed to patient safety. As with Victoza® and other GLP- 1 receptor agonists, Novo Nordisk proposed that Saxenda® should include a boxed warning regarding the potential risk of MTC in its US product label as well as in its EU label11.
Novo Nordisk is committed to patient safety and continuously monitors the safety profile of liraglutide.
Based on the totality of available data, there is no indication of a causal relationship between Saxenda® (liraglutide 3 mg) and breast cancer.
Based on the overall and limited number of reported events from the SCALETM clinical development programme, there was a numerical imbalance in the number of cases of breast cancer in females with obesity:3
Saxenda®-treated: 14 events, equating to 0.6% of the trial population.
Placebo-treated: 3 events, equating to 0.2% of the trial population.
Over one third of the females with breast cancer had a relevant medical history (previous breast neoplasm or cancer, family history of breast cancer or history of hormone replacement therapy).12
The numerical excess of breast cancer is not supported by non-clinical data or post-marketing experience with liraglutide (1.8 mg). A numerical imbalance does not necessarily indicate an increased risk.
Novo Nordisk is committed to patient safety and continuously monitors the safety profile of Saxenda®.
To further explain the higher incidence:
The majority of breast cancers detected within the SCALETM clinical development programme were diagnosed within one year of starting treatment. Given the current understanding of breast tumour growth rates, the size/stage of the reported breast cancers, and the short interval between randomisation and breast cancer diagnosis, it appears likely that the majority of the invasive cancers reported in both liraglutide and placebo arms had been present (but undiagnosed) prior to study entry.3
In the SCALETM clinical development programme, Saxenda®-treated women with events of breast neoplasms generally experienced greater than the average weight loss compared with placebo. It raises the possibility that weight loss may have led to enhanced detection of breast neoplasms in this group of women, as women who have obesity are also less likely to go for routine mammography.13 As well, women with obesity are at a higher risk of developing certain types of neoplasms (benign and cancerous), including post-menopausal breast cancer.14-17
There was no imbalance seen for overall neoplasms in the SCALETM clinical development programme for Saxenda® compared with placebo treatment.3
The warning and precaution around hypoglycaemia in the Saxenda® US and EU label specifically relates to the use of Saxenda® in people with obesity and type 2 diabetes3.
US label: Saxenda® Prescribing Information
Under Section 5.4 ‘Risk for Hypoglycemia with Concomitant Use of Anti- Diabetic Therapy’, the label states:52
‘The risk for serious hypoglycemia is increased when Saxenda is used in combination with insulin secretagogues (for example, sulfonylureas) in patients with type 2 diabetes mellitus. Therefore, patients may require a lower dose of sulfonylurea (or other concomitantly administered insulin secretagogues) in this setting. Saxenda should not be used in patients taking insulin.
EU label: Saxenda® Summary of Product Characteristics
Under Section 4.4 ‘Special warnings and precautions for use, the label states:50
‘Patients with type 2 diabetes mellitus receiving liraglutide in combination with a sulphonylurea may have an increased risk of hypoglycaemia. The risk of hypoglycaemia may be lowered by a reduction in the dose of sulphonylurea. The addition of Saxenda® in patients treated with insulin has not been evaluated.
Saxenda® has not been investigated in people with type 2 diabetes treated with insulin.
NOTE: LOCAL LABEL MAY DIFFER.
Additional Information:
In the SCALE(TM) Diabetes trial, participants were using oral antidiabetic drugs to control blood glucose during the study. Participants were treated with metformin, sulfonylurea or glitazone as single-agent therapy or any combination of these compounds (metformin+sulfonylurea, metformin+glitazone, sulfonylurea+glitazone, metformin+sulfonylurea+glitazone). A maximum of 30% of adults in the trial were treated with sulfonylureas.3
Severe hypoglycaemia (requiring third-party assistance) was reported by 0.7% of people treated with Saxenda® and only in those people concomitantly treated with sulfonylurea. Among people not concomitantly treated with sulfonylurea, 15.7% of people treated with Saxenda® and 7.6% of people treated with placebo reported documented symptomatic hypoglycaemic events (plasma glucose 3.9 mmol/L accompanied by symptoms). In people concomitantly treated with sulfonylurea, documented symptomatic hypoglycaemia was reported by 43.6% of people treated with Saxenda® and by 27.3% of people treated with placebo.3
Obesity is a chronic disease that requires long-term management.
It is a complex and multifactorial disease that is influenced by physiological, psychological, environmental, socio-economic and genetic factors.18
Obesity is recognized by health organizations including the World Health Organization (WHO) and the American Medical Association,19-21 and is defined as excess fat storage, leading to impaired health.22
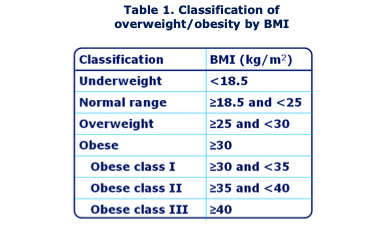
Obesity is commonly measured using body mass index (BMI), which is an estimate of an individual’s body fat in relation to their weight and height (see Table 1).18 Obesity is classified as BMI 30 kg/m2 and is further sub- divided into three classes (Class I, II, III) depending on the increase in BMI above 30 kg/m2.
BMI does have its limitations–it does not differentiate fat mass from fat-free mass (e.g. muscle) and can therefore lead to an over- or under-estimation of body fat in some individuals.23 Alternative methods of body-fat estimation include measurement of waist circumference and waist-to-hip ratio.
Obesity is a chronic disease that requires long-term management. It has many serious health consequences and is associated with a decreased life expectancy. The risk of morbidity and mortality increases with the severity of obesity.24, 25 It is a complex and multifactorial disease that is influenced by genetic, physiological, environmental, and psychological factors.
Obesity is associated with serious comorbidities, including type 2 diabetes, heart disease, increased blood pressure, cholesterol and triglyceride levels, obstructive sleep apnoea, and certain types of cancer.26-28 More than half of people with a BMI of 35 or higher (Class II and III obesity) have elevated glucose levels.29 Regardless of initial body weight, a sustained weight loss of 5–10% in people with obesity has significant health benefits30 and has been proven to delay and/or prevent type 2 diabetes.31, 32
In June 2013, the American Medical Association (AMA), the largest physician group in the US, officially recognised obesity as a disease.21
The classification of obesity as a disease was based on a review of the following disease characteristics:33
- “An impairment of the normal functioning of some aspect of the body
- Characteristic signs or symptoms; and
- Resultant harm or morbidity to the entity affected”.
This new classification of obesity was made to change the way the medical community manages people with obesity and to improve health outcomes, especially in those with obesity-related comorbidities.21 This policy change has since been acknowledged by other leading professional and patient associations, including the World Health Organization.
Obesity occurs when sustained caloric intake exceeds expenditure. Since this energy imbalance can be caused by genetic, physiological, environmental, and psychological factors, its impact on weight gain may differ from one person to another. Emerging science has identified some important physiological factors that may play central roles in obesity development, such as appetite control systems and early life experiences.
In June 2013, the American Medical Association (AMA), the largest physician group in the US, officially recognized obesity as a disease to change the way the medical community manages people with obesity and to improve health outcomes, especially in those with obesity-related comorbidities.21 This policy change by the AMA was an important step to improve obesity care, and it has been acknowledged by other leading professional and patient associations, including the World Health Organization.
Obesity is, however, often still portrayed as a lifestyle choice rather than a disease because we can choose what to eat. However, we can also choose to not smoke, to exercise, or to drink less – yet lung cancer, cardiovascular disease, and cirrhosis are recognised as diseases.
Obesity is associated with serious comorbidities, including type 2 diabetes, heart disease, increased blood pressure, cholesterol and triglyceride levels, obstructive sleep apnoea, and certain types of cancer. 24,34-36 More than half of people with a BMI of 35 kg/m2 or higher (Class II and III obesity) have elevated glucose levels.29 Regardless of initial body weight, a sustained weight loss of 5–10% in people with obesity has significant health benefits and has been proven to delay and/or prevent type 2 diabetes.37-39
A weight loss of 5–10% in people with obesity has significant health benefits, including improvements in blood glucose levels, blood pressure, cholesterol levels40 and obstructive sleep apnoea32, 41-43 and health-related quality of life.44, 45
Despite increased public focus on obesity, the disease remains substantially under-diagnosed and under-reported46, 47 and it is estimated that less than 23% of US patients with a BMI of between 35 and 40 kg/m2 are reported as being obese.47 Counseling rates of obesity also remain low among physicians in the US and there is a need to improve obesity care.48
Healthy eating and physical activity must be part of any weight loss intervention, but this is not always sufficient to maintain weight loss. Maintaining weight loss is challenging, partly due to metabolic changes in the body.49, 50
Due to the complexity of obesity there is a need for multiple treatment options that can help people with obesity to lose weight and keep it off and thereby improve their health.34
Type 2 diabetes is a common comorbidity for people with obesity.18 The underlying mechanisms of association are not clearly defined; however, insulin resistance, which is frequently seen in people with obesity, plays a key role.35 Excess fat tissue around the abdomen is also a major risk factor.51
Obesity directly affects the cardiovascular system and is associated with numerous heart complications, including congestive heart failure, arrhythmia (abnormal heart rhythm), coronary artery disease and stroke. Specifically, obesity increases the risk of heart disease through:52
- Increased blood cholesterol and triglyceride levels
- Decreased high-density lipoprotein (HDL; ‘good’) cholesterol
- Increased blood pressure
- Glucose intolerance/diabetes
- Obstructive sleep apnoea53
Obesity and overweight are also independent risk factors for myocardial infarction (heart attack) and ischemic heart disease (coronary heart disease),54 the leading cause of death worldwide.55
Hypertension (high blood pressure) is a common comorbidity of obesity and the link is well established. People with obesity have a greater prevalence of high blood pressure compared with people who do not have obesity. The underlying mechanism through which obesity directly causes hypertension is not clearly understood.56
Dyslipidaemia (abnormal lipid profile) is a common comorbidity of obesity. Obesity has an impact on blood lipids, resulting in increased cardiovascular risk. Typically, people with obesity have increased fasting plasma triglycerides, high low-density lipoprotein cholesterol and low HDL (‘good’) cholesterol.56, 57
OSA is a common sleep-related breathing disorder that affects 5-10 millions (2-4%) adults in the US, and around 5 million adults in Europe.41,58 It is characterized by a decrease or total arrest in airflow during breathing. Symptoms of OSA include daytime sleepiness, impaired concentration, nocturia (the need to wake at night to urinate), decreased libido, restless sleep and snoring.41
The most common cause of OSA is overweight and obesity: 60–90% of people with OSA are either overweight or have obesity, and people with obesity (BMI >29 kg/m2) are at a high risk of developing OSA.59 Each BMI unit increase is associated with a 14% increased risk of developing OSA.60
OSA is also associated with an increased risk of hypertension, stroke, insulin resistance, metabolic syndrome, type 2 diabetes, decreased quality of life, increased risk of vehicle accidents and increased mortality.60-63
The severity of OSA is commonly measured using the apnoea-hypopnoea index (AHI).64 This averages the combined number of apnoeas (complete pauses in breathing) and hypopnoeas (partial reduction in breathing) lasting at least 10 seconds that occur per hour of sleep.
- AHI severity categories:65
- None: ≤4.9 events per hour of sleep
- Mild: 5.0–14.9 events per hour of sleep
- Moderate: 15.0–29.9 events per hour of sleep
- Severe: ≥30.0 events per hour of sleep
Obesity is associated with several respiratory complications, including OSA, due to an increased demand for ventilation and breathing workload, decreased ventilation reserve capacity and closure of peripheral lung units.66
People with obesity also have excess fat around the neck and chest, which places strain on the throat and chest when the muscles are relaxed during sleep. Fatty deposits can also build up within the throat, narrowing the air passage. These factors can impair, or completely stop, airflow during sleep.59
It is recommended that people who are overweight or with obesity diagnosed with OSA should be encouraged to lose weight. It is believed that weight loss in people with OSA can lead to improvements in their symptoms67.
Glucagon-like peptide-1 (GLP-1) is a naturally occurring hormone that is released in response to food intake. It regulates appetite and food intake by decreasing hunger and increasing feelings of fullness and satiety after eating68, 69.
GLP-1 also plays an important role in maintaining a normal level of glucose in the blood by stimulating the release of insulin and suppressing glucagon secretion in a glucose-dependent manner.70
Appetite is defined as the desire to consume food and it manifests as the physical feeling of hunger. Appetite regulation is a complex process involving multiple hormones, which relay signals between the gut and the brain71.
Appetite regulation is important to maintain a healthy body weight, with hunger ensuring that people consume sufficient nutrients to match the demands of the body. Dysregulation of appetite can lead to malnutrition or obesity, both of which have severe health consequences72.
Both the brain and gut play crucial roles in appetite regulation. After a meal, the gut detects and responds to the presence of food by producing several hormones/peptides, such as ghrelin, leptin and GLP-1. They are processed by the brain and translated into feelings of satiety or hunger and thereby control food intake4.
Saxenda® Summary of Product Characteristics (Prescribing Information)
Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ, et al. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. Journal of medicinal chemistry. 2000;43(9):1664-9.
T-Cells C. Novo Nordisk Receives FDA Approval of Saxenda® (liraglutide) injection 3 mg Label Update Including Long-Term Safety and Efficacy Data from 3-Year Trial.
Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307(5717):1909-14.
Divino V, Boye KS, Lebrec J, DeKoven M, Norrbacka K. GLP-1 RA treatment and dosing patterns among type 2 diabetes patients in six countries: a retrospective analysis of pharmacy claims data. Diabetes Therapy. 2019;10(3):1067-88.
Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. Jama. 2014;311(1):74-86.
Egan AG, Blind E, Dunder K, de Graeff PA, Hummer BT, Bourcier T, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. New England Journal of Medicine. 2014;370(9):794-7.
Pedersen S, DeFronzo RA, Bergenstal R, Bode B, Kushner R, Lewin A, et al. Effects of liraglutide 3.0 mg and 1.8 mg on body weight and cardiometabolic risk factors in adults with overweight or obesity and type 2 diabetes (T2D): The SCALE diabetes randomized, double-blind, placebo-controlled, 56-week trial. Canadian Journal of Diabetes. 2015;39:S36.
O'Neil PM, Garvey WT, Gonzalez-Campoy JM, Mora P, Ortiz RV, Guerrero G, et al. Effects of liraglutide 3.0 mg on weight and risk factors in hispanic versus non-hipanic populations: subgroup analysis from scale randomized trials. Endocrine Practice. 2016;22(11):1277-87.
Iepsen EW, Torekov SS, Holst JJ. Liraglutide for type 2 diabetes and obesity: a 2015 update. Expert review of cardiovascular therapy. 2015;13(7):753-67.
Fala L. Tanzeum (Albiglutide): a once-weekly GLP-1 receptor agonist subcutaneous injection approved for the treatment of patients with type 2 diabetes. American health & drug benefits. 2015;8(Spec Feature):126.
Chawla A, Carls G, Deng E, Tuttle E. The expected net present value of developing weight management drugs in the context of drug safety litigation. Pharmacoeconomics. 2015;33(7):749-63.
Maruthur NM, Bolen S, Brancati FL, Clark JM. Obesity and mammography: a systematic review and meta-analysis. Journal of general internal medicine. 2009;24(5):665-77.
Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, et al. Dual effects of weight and weight gain on breast cancer risk. jama. 1997;278(17):1407-11.
Michels KB, Terry KL, Willett WC. Longitudinal study on the role of body size in premenopausal breast cancer. Archives of internal medicine. 2006;166(21):2395-402.
Tehard B, Lahmann PH, Riboli E, Clavel‐Chapelon F. Anthropometry, breast cancer and menopausal status: Use of repeated measurements over 10 years of follow‐up—results of the French E3N women's cohort study. International journal of cancer. 2004;111(2):264-9.
Health NIo. National Cancer Institute. Obesity and Cancer Risk Fact Sheet. 2013.
Pi-Sunyer FX, Becker DM, Bouchard C, Carleton RA, Colditz GA, Dietz WH, et al. Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Journal of the American Dietetic Association. 1998;98(10):1178-91.
Garvey WT, Mechanick JI, Einhorn D. The American Association of Clinical Endocrinologists and the American College of Endocrinology: 2014 advanced framework for a new diagnosis of obesity as a chronic disease. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2014;20(9):977.
Obesity P. Managing the Global Epidemic. World Health Organization (WHO), Genf. 1998.
Association AM, editor Declaration to classify obesity as a disease. Annual Meeting Report; 2013.
Souza MCCd, Tibúrcio JD, Bicalho JMF, Rennó HMdS, Dutra JS, Campos LG, et al. Factors associated with obesity and overweight in school-aged children. Texto & Contexto-Enfermagem. 2014;23:712-9.
Dulloo AG, Jacquet J, Solinas G, Montani J-P, Schutz Y. Body composition phenotypes in pathways to obesity and the metabolic syndrome. International journal of obesity. 2010;34(2):S4-S17.
Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC public health. 2009;9(1):1-20.
Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Mamun AA, Bonneux L, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Annals of internal medicine. 2003;138(1):24-32.
Hopman WM, Berger C, Joseph L, Barr SI, Gao Y, Prior JC, et al. The association between body mass index and health-related quality of life: data from CaMos, a stratified population study. Quality of life research. 2007;16(10):1595-603.
Torekov S, Madsbad S, Holst J. Obesity–an indication for GLP‐1 treatment? Obesity pathophysiology and GLP‐1 treatment potential. obesity reviews. 2011;12(8):593-601.
Overgaard RV, Petri KC, Jacobsen LV, Jensen CB. Liraglutide 3.0 mg for weight management: a population pharmacokinetic analysis. Clinical pharmacokinetics. 2016;55(11):1413-22.
Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. American heart journal. 2000;140(1):98-104.
Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Journal of the American college of cardiology. 2014;63(25 Part B):2985-3023.
Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine. 2001;344(18):1343-50.
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. 2002.
Jarris PE. Obesity as disease: an opportunity for integrating public health and clinical medicine. Journal of Public Health Management and Practice. 2013;19(6):610-2.
Ferguson C, David S, Divine L, George Washington University SoPH, Health Services DoHP. Obesity drug outcome measures. A consensus report of considerations regarding pharmacologic intervention. Department of Health Policy, School of Public Health and Health Services, The George Washington University. 2012.
Eckel RH, Kahn SE, Ferrannini E, Goldfine AB, Nathan DM, Schwartz MW, et al. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? The Journal of Clinical Endocrinology & Metabolism. 2011;96(6):1654-63.
Khaodhiar L, Cummings S, Apovian CM. Treating diabetes and prediabetes by focusing on obesity management. Current diabetes reports. 2009;9(5):348-54.
Mertens IL, Van Gaal LF. Overweight, obesity, and blood pressure: the effects of modest weight reduction. Obesity research. 2000;8(3):270-8.
PI-SUNYER EX. Weight loss and mortality in type 2 diabetes. Diabetes care. 2000;23(10):1451-.
Group LAR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Archives of internal medicine. 2010;170(17):1566-75.
Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes care. 2011;34(7):1481-6.
Foster GD, Borradaile KE, Sanders MH, Millman R, Zammit G, Newman AB, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Archives of internal medicine. 2009;169(17):1619-26.
Kuna ST, Reboussin DM, Borradaile KE, Sanders MH, Millman RP, Zammit G, et al. Long-term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep. 2013;36(5):641-9.
Dattilo AM, Kris-Etherton P. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. The American journal of clinical nutrition. 1992;56(2):320-8.
Warkentin L, Das D, Majumdar S, Johnson J, Padwal R. The effect of weight loss on health‐related quality of life: systematic review and meta‐analysis of randomized trials. Obesity Reviews. 2014;15(3):169-82.
Wright F, Boyle S, Baxter K, Gilchrist L, Nellaney J, Greenlaw N, et al. Understanding the relationship between weight loss, emotional well-being and health-related quality of life in patients attending a specialist obesity weight management service. Journal of Health Psychology. 2013;18(4):574-86.
Crawford AG, Cote C, Couto J, Daskiran M, Gunnarsson C, Haas K, et al. Prevalence of obesity, type II diabetes mellitus, hyperlipidemia, and hypertension in the United States: findings from the GE Centricity Electronic Medical Record database. Population health management. 2010;13(3):151-61.
Ma J, Xiao L, Stafford RS. Adult obesity and office‐based quality of care in the United States. Obesity. 2009;17(5):1077-85.
Kraschnewski JL, Sciamanna CN, Stuckey HL, Chuang CH, Lehman EB, Hwang KO, et al. A silent response to the obesity epidemic: decline in US physician weight counseling. Medical care. 2013:186-92.
Mann T, Tomiyama AJ, Westling E, Lew A-M, Samuels B, Chatman J. Medicare's search for effective obesity treatments: diets are not the answer. American Psychologist. 2007;62(3):220.
MacLean PS, Wing RR, Davidson T, Epstein L, Goodpaster B, Hall KD, et al. NIH working group report: innovative research to improve maintenance of weight loss. Obesity. 2015;23(1):7-15.
Björntorp P. Metabolic implications of body fat distribution. Diabetes care. 1991;14(12):1132-43.
Mathew B, Francis L, Kayalar A, Cone J. Obesity: effects on cardiovascular disease and its diagnosis. The Journal of the American Board of Family Medicine. 2008;21(6):562-8.
Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. Jama. 2003;290(14):1906-14.
Thomsen M, Nordestgaard BG. Myocardial infarction and ischemic heart disease in overweight and obesity with and without metabolic syndrome. JAMA internal medicine. 2014;174(1):15-22.
Organization WH. The global burden of disease: 2004 update: World Health Organization; 2008.
Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity-induced hypertension. Hypertension research. 2010;33(5):386-93.
Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. Jama. 2014;311(8):806-14.
Decramer M, Sibille Y, Bush A, Carlsen K, Rabe K, Clancy L, et al. The European Union conference on chronic respiratory disease: purpose and conclusions. Eur Respiratory Soc; 2011. p. 738-42.
Pillar G, Shehadeh N. Abdominal fat and sleep apnea: the chicken or the egg? Diabetes care. 2008;31(Supplement_2):S303-S9.
Zhao J, Xu W, Yun F, Zhao H, Li W, Gong Y, et al. Chronic obstructive sleep apnea causes atrial remodeling in canines: mechanisms and implications. Basic research in cardiology. 2014;109(5):1-13.
Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. European heart journal. 2004;25(9):735-41.
Ip MS, Tse H-F, Lam B, Tsang KW, Lam W-K. Endothelial function in obstructive sleep apnea and response to treatment. American journal of respiratory and critical care medicine. 2004;169(3):348-53.
Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31(8):1079-85.
Medicine AOSATFotAAoS. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. Journal of clinical sleep medicine. 2009;5(3):263-76.
Sutherland K, Takaya H, Qian J, Petocz P, Ng AT, Cistulli PA. Oral appliance treatment response and polysomnographic phenotypes of obstructive sleep apnea. Journal of Clinical Sleep Medicine. 2015;11(8):861-8.
Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898-918.
Tuomilehto H, Seppä J, Uusitupa M. Obesity and obstructive sleep apnea–clinical significance of weight loss. Sleep medicine reviews. 2013;17(5):321-9.
Ørskov C, Wettergren A, Holst J. Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scandinavian journal of gastroenterology. 1996;31(7):665-70.
Flint A, Raben A, Ersbøll A, Holst J, Astrup A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. International journal of obesity. 2001;25(6):781-92.
Edwards C, Todd JF, Mahmoudi M, Wang Z, Wang RM, Ghatei MA, et al. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9-39. Diabetes. 1999;48(1):86-93.
Heisler LK, Lam DD. An appetite for life: brain regulation of hunger and satiety. Current opinion in pharmacology. 2017;37:100-6.
Arora S. Role of neuropeptides in appetite regulation and obesity–a review. Neuropeptides. 2006;40(6):375-401.